A single protein can be identified in a cell lysate that contains thousands of different proteins and its abundance quantified through western blot (WB) analysis. This could be achieved by two steps.
This How to Guide cover the following content:
Sample Preparation
Sample preparation is isolating the protein from a source, normally cells or tissues. After cell lysis, proteins exist in the kept supernatant. Then, the protein concentration is determined and loading buffer is added to the protein suspension.
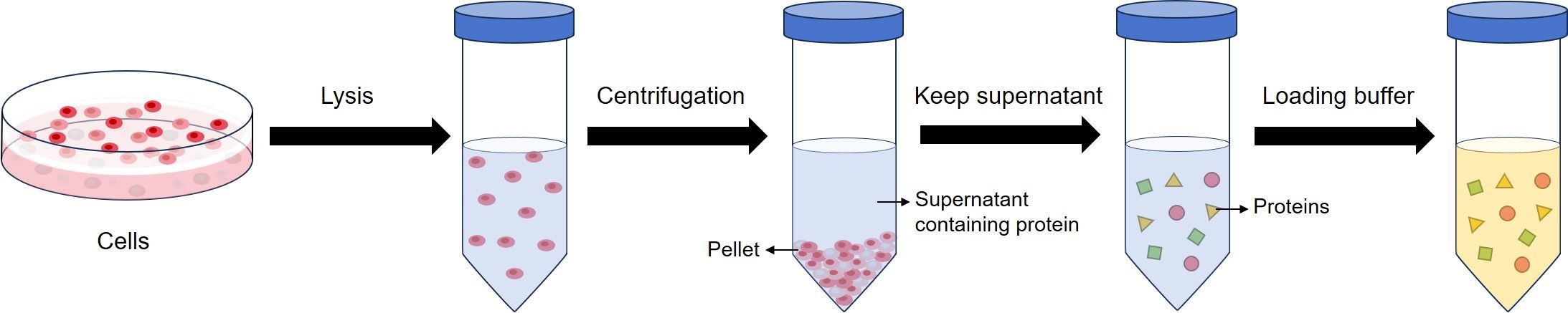
Normally, loading buffer used for WB contains sodium dodecyl sulfate (SDS), and beta-mercaptoethanol (BME). SDS disrupts the secondary, tertiary and quaternary structure of the protein to produce a linear polypeptide chain coated with negatively charged SDS molecules. BME contributes with protein denaturation by reducing all disulfide bonds.
 Fig.1 Detergent molecules coat the unfolded protein chain.1
Fig.1 Detergent molecules coat the unfolded protein chain.1
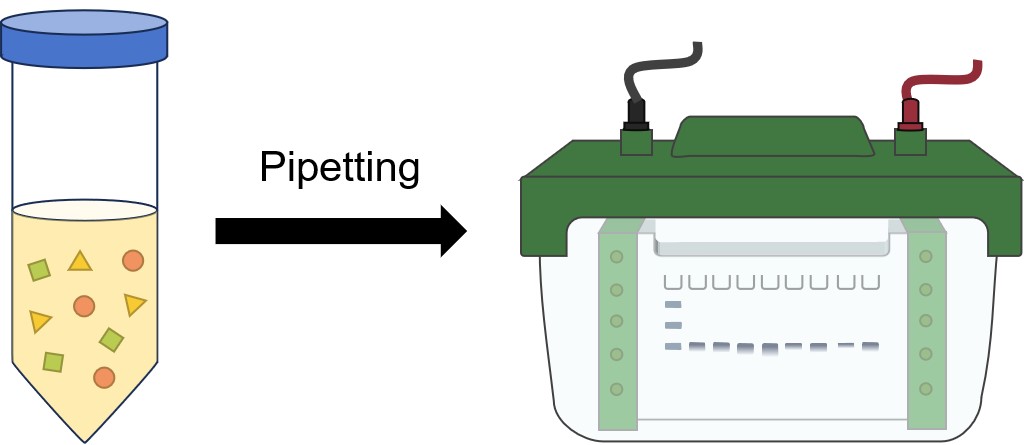
SDS-PAGE
The first step is placing the protein sample in a well on top of the gel. A ladder is usually loaded in one of the wells. Ladders are protein mixtures of known molecular weight, that allow the researcher to determine the molecular weights of the other proteins on the gel. Once protein ladder and all the sample(s) are loaded, a current is run across the gel. Since the protein is bound to negatively charged SDS, it is pulled down through the gel to the positive pole. The larger the protein, the slower it moves.
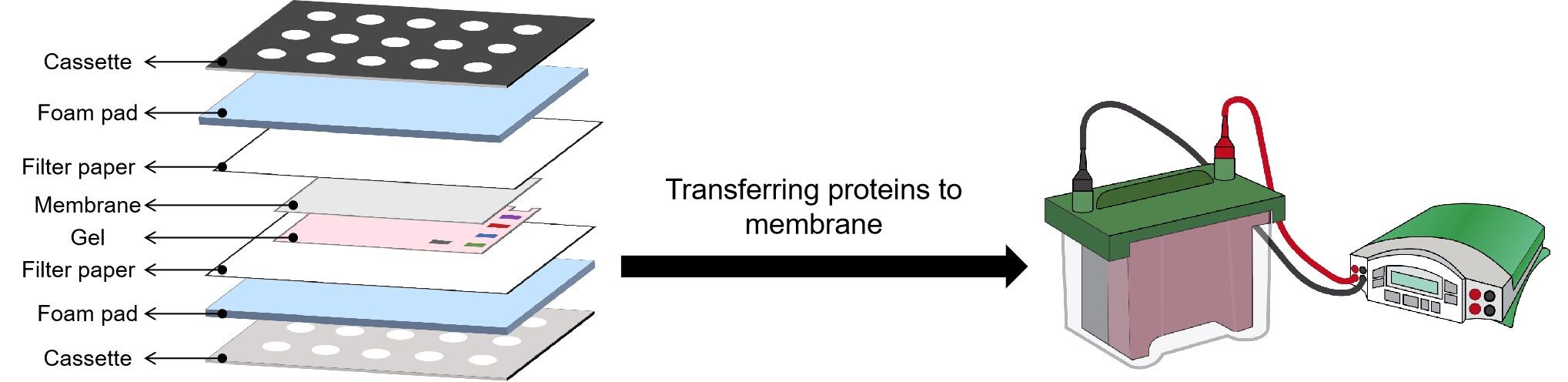
Protein Transfer
After the gel is run, it is placed against a membrane, and current is passed across the gel to the membrane, transferring the proteins onto the membrane. The components and order of the “sandwich” are shown in the figure.
Immunoblotting
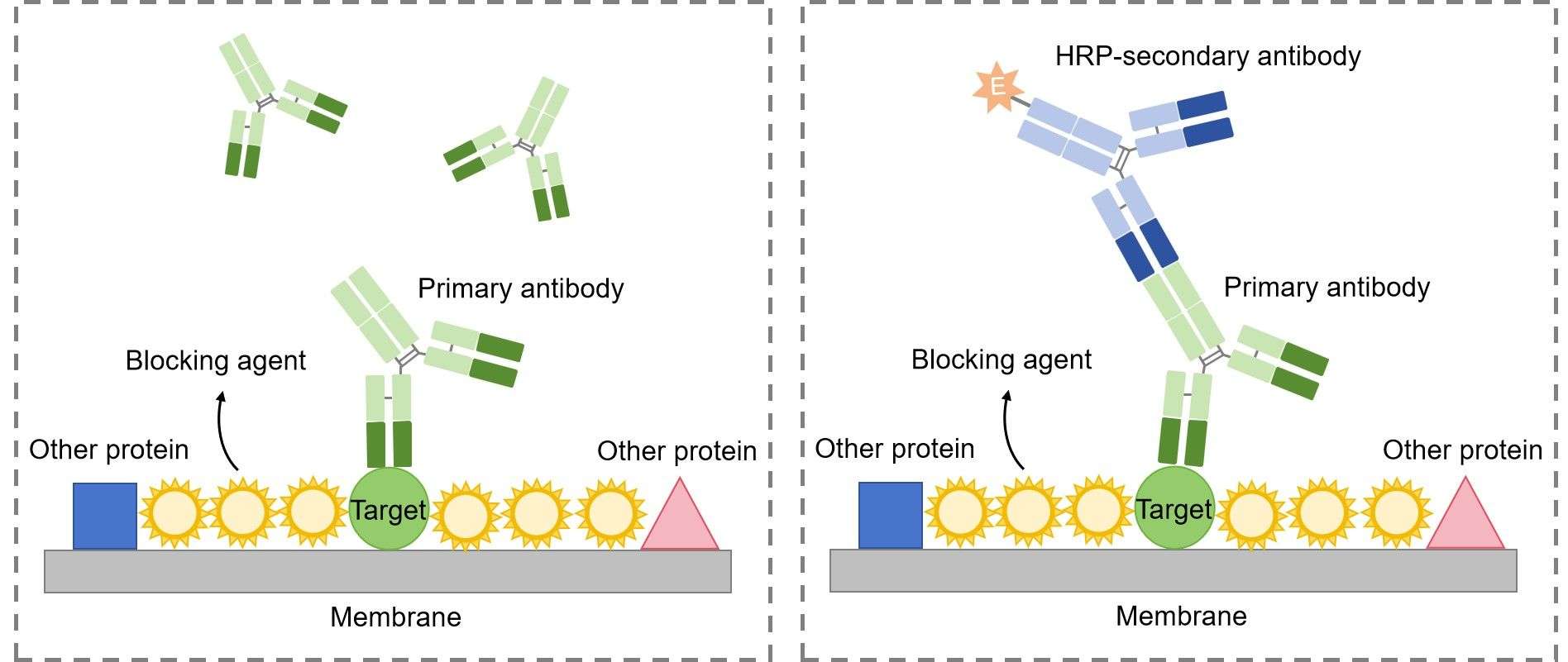
The first step in immunoblotting is to wash the membrane and block it with non-specific protein. The non-specific protein binds to the surface of the membrane where protein is not already present. This will prevent antibody from binding to the membrane and giving a non-specific signal.
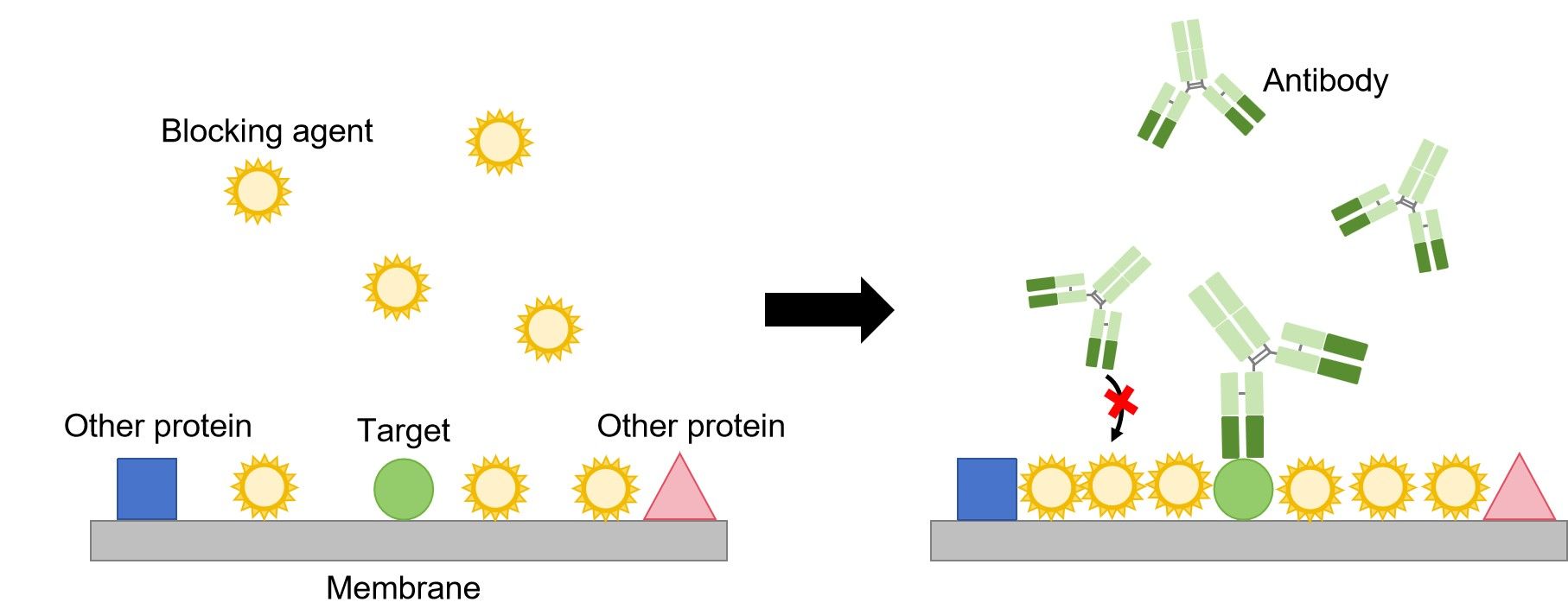
Then, the primary antibody is added to the solution in which the membrane is floating. The primary antibody recognizes a specific amino-acid sequence of a particular protein. After adequate washing to remove unbound primary antibody, secondary antibody is added. Secondary antibody recognizes the primary antibody, and usually is conjugated with an enzyme, such as HRP (Horse Radish Peroxidase).
Detection
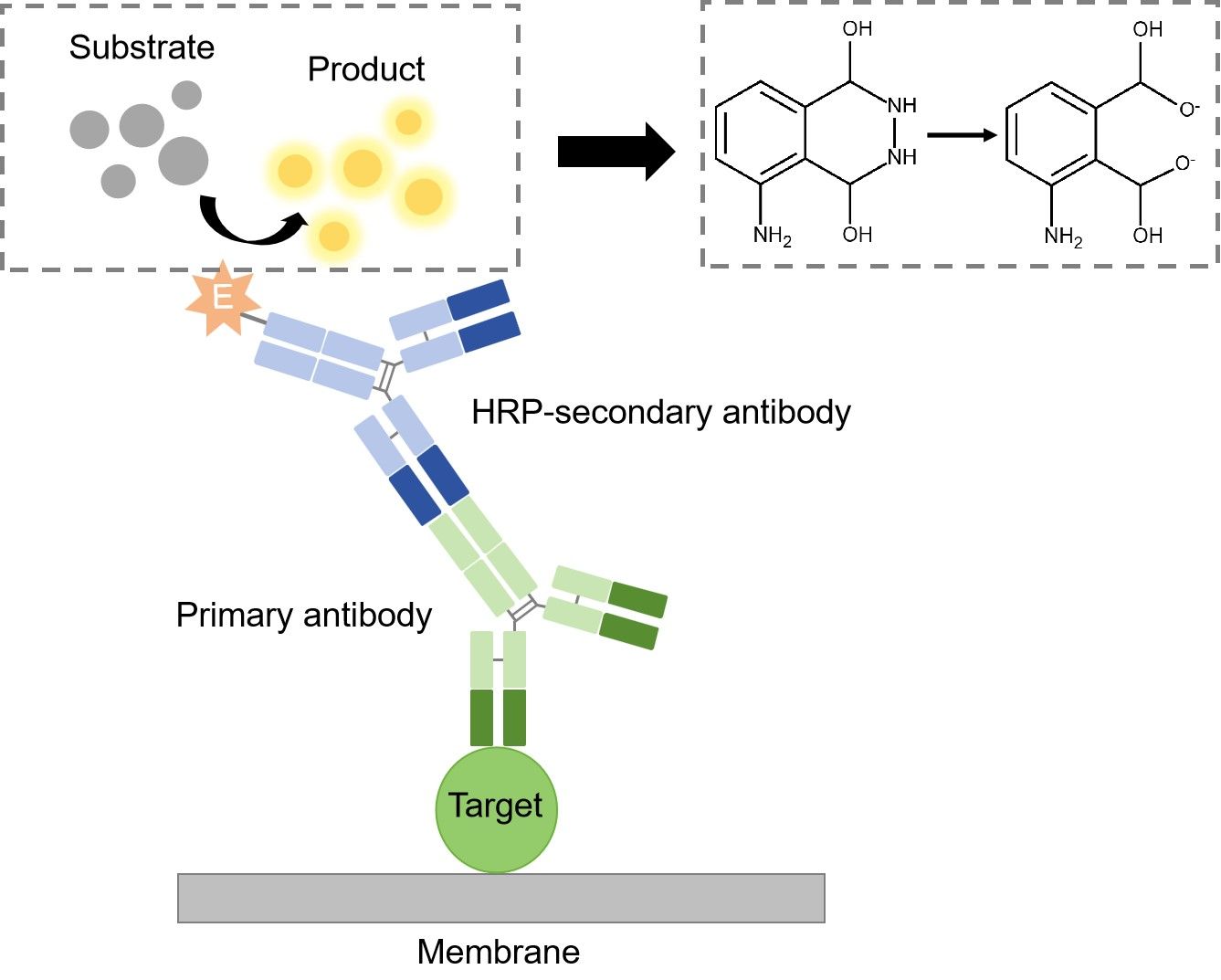
The detection method used is dependent upon the enzyme to which the secondary antibody is conjugated. The most common enzyme used in WB is HRP, and the substrate used for detection is known as chemiluminescent substrate. Once the substrate has been added, the light being emitted can be detected with film or a photo imager.
More WB Resource
Western Blot Protocols & Troubleshooting & Guide
Sample Preparation in Western Blot Assay
Protein Transfer from Gel to Membrane in Western Blot Assay
Running an SDS-PAGE Gel in Western Blot Assay
Stripping and Reprobing Protocol
Reference